The binding energies of hydrogen, oxygen, water and peroxide molecules... | Download Scientific Diagram

The binding energy of `e^(-)` in ground state of hydrogen atom is 13.6 eV . The energies required to - YouTube

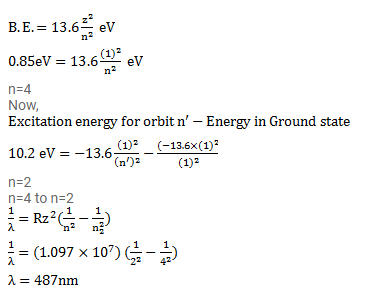
A hydrogen atom in a state having a binding energy of 0.85 eV makes transition to a state with - YouTube
If the binding energy of the electron in the ground state of hydrogen atom is E, then the frequency of electron in the nth orbit is

The binding energy for the nth hydrogen atom by the most stable vac +... | Download Scientific Diagram
If the binding energy of the electron in the ground state of hydrogen atom is E, then the frequency of electron in the nth orbit is